Signal Transduction Group
PD Dr. Malte Kriegs, Dipl.-Biochem.
Dr. Nina Struve, Dipl. Biochem.
Konstantin Hoffer, BTA
Britta Riepen, MTA
Alicia Eckhardt, PhD Stud
Participation & Membership
AG3 is part of the University Cancer Center Hamburg (UCCH) and the Hubertus Wald-Tumorzentrum. The projects of AG3 are part of the UCCH research programs Head & Neck Cancer and Neuro-Oncology. The Group is also funded by the Mildred Scheel Cancer Career Center HaTriCS4 at the UKE.
AG3 is part of the graduate school "Innovative Technologies in Cancer Diagnostics and Therapy" (www.uke.de/kliniken-institute/zentren/universit%C3%A4res-cancer-center-hamburg-(ucch)/forschung/grk-innovative-technologien/index.html
AG3 runs the UCCH Kinomics Core Facility (see below)
Scientists of AG3 are members in the European Society for Radiotherapy & Oncology (ESTRO), Deutsche Gesellschaft für Radioonkologie (DEGRO), Deutsche Gesellschaft für Biologische Strahlenforschung (DeGBS), Society for Neuro-Oncology (SNO), Deutsche Gesellschaft für Hals-, Nasen- und Ohrenheilkunde (DGHNO), Hamburger Cancer Society
Interdisciplinary research topic 1 (PI Kriegs)
Signal transduction in head & neck cancer (HNSCC) and personalized therapy
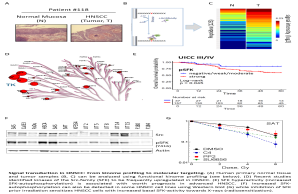
The aim of this project is to analyze the kinase dependent signal transduction in head & neck cancer to identify tumor specific changes and changes induced by different therapy modalities such as X-rays. Therefore, we analyze established HNSCC cell lines and primary tumor tissue using classical biochemical methods, translational approaches such as tissue micro arrays as well as cutting-edge technologies like differential proteomics and functional kinome profiling (see also below UCCH Kinomics Core Facility). By understanding the signal transduction and biology of HNSCC we aim to use selected kinase inhibitors for individualized therapy approaches. A special focus is on the combination of specific targeting strategies with X-rays to induce radiosensitization.
Signal transduction in HNSCC: From kinome profiling to molecular targeting. (A) Human primary normal tissue and tumor samples (B, C) can be analyzed using functional kinome profiling (see below). (D) Recent studies identified kinases of the Src-family (SFK) to be frequently upregulated in HNSCC. (E) SFK hyperactivity (increased SFK-autophosphorylation) is associated with worth prognosis in advanced HNSCC. (F) Increased SFK-autophosphorylation can also be detected in some HNSCC cell lines using Western blot (G) while inhibition of SFK prior irradiation sensitizes HNSCC cells with increased basal SFK-activity towards X-rays (radiosensitization).
Contact: [email protected]
See also: https://www.uke.de/kliniken-institute/kliniken/hals-nasen-und-ohrenheilkunde/forschung/arbeitsgruppen/index.html
Collaborations: Department of Otorhinolaryngology, Institute of Anatomy and Experimental Morphology, Institute of Pathology, Institute of Biochemistry and Signal Transduction, Department of Oncology & Hematology (II. Med.), Institute of Clinical Chemistry and Laboratory Medicine, Kinderkrebs-Zentrum Hamburg
Grants: Federal Ministry of Education and Research (BMBF); Hamburger Krebsgesellschaft; Werner Otto-Stiftung; Stiftung Tumorforschung Kopf-Hals; Hamburger Stiftung zur Förderung der Krebsbekämpfung; Forschungsförderungsfonds des Fachbereichs Medizin; Spierling-Stiftung
Publications:
Wurlitzer M, Möckelmann N, Kriegs M, Vens M, Omidi M, Hoffer K, Bargen CV, Möller-Koop C, Witt M, Droste C, Oetting A, Petersen H, Busch CJ, Münscher A, Schlüter H, Clauditz TS, Rieckmann T.Mass Spectrometric Comparison of HPV-Positive and HPV-Negative Oropharyngeal Cancer. Cancers. 2020 Jun 11;12(6):1531..
- Hintelmann K, Kriegs M, Rothkamm K, Rieckmann T. Improving the Efficacy of Tumor Radiosensitization Through Combined Molecular Targeting. Front Oncol. 2020, 10:1260. (review)
- Beizaei K, Gleißner L, Hoffer K, Bußmann L, Vu AT, Steinmeister L, Laban S, Möckelmann N, Münscher A, Petersen C, Rothkamm K, Kriegs M. Receptor tyrosine kinase MET as potential target of multi-kinase inhibitor and radiosensitizer sorafenib in HNSCC. Head Neck. 2019 Jan;41(1):208-215.
- Kriegs M, Clauditz T, Bußmann L, Hoffer K, Bartels J, Buhs S, Gerull H, Rieckmann T, Petersen C, Betz CS, Rothkamm K, Nollau P, Münscher A. Analyzing expression and phosphorylation of EGF-receptor in HNSCC. Sci Rep. 2019 Sep 19;9(1):13564.
- Rieckmann T, Rothkamm K, Kriegs M. The failure of cetuximab-based de-intensified regimes for HPV-positive OPSCC: a radiobiologists` perspective. Clin Transl Radiat Oncol. 2019, May 22; 17:47-50. (Commentary)
- Gleißner L, Kwiatkowski M, Myllynen L, Steffen P, Petersen C, Rothkamm K, Schlüter H, Kriegs M. Analyzing the influence of kinase inhibitors on DNA repair by differential proteomics of chromatin-interacting proteins and nuclear phospho-proteins. Oncotarget. 2017 Nov 10;8(67): 110983-110993.
- Braig F, Kriegs M, Voigtlaender M, Habel B, Grob T, Biskup K, Blanchard V, Sack M, Thalhammer A, Ben Batalla I, Braren I, Laban S, Danielczyk A, Goletz S, Jakubowicz E, Märkl B, Trepel M, Knecht R, Riecken K, Fehse B, Loges S, Bokemeyer C, Binder M. Cetuximab Resistance in Head and Neck Cancer Is Mediated by EGFR-K521 Polymorphism. Cancer Res, 2017 Mar 1;77(5):1188-1199.
- Busch CJ, Kröger MS, Jensen J, Kriegs M, Gatzemeier F, Petersen C, Münscher A, Rothkamm K, Rieckmann T. G2-checkpoint targeting and radiosensitization of HPV/p16-positive HNSCC cells through the inhibition of Chk1 and Wee1. Radiother Oncol, 2017 Feb;122(2):260-266.2016,
- Möckelmann N, Kriegs M, Lörincz BB, Busch CJ, Knecht R. Molecular targeting in combination with platinum-based chemoradiotherapy in head and neck cancer treatment. Head Neck. 2016, Apr;38 Suppl 1:E2173-81 (review)
- Kriegs M, Kasten-Pisula U, Riepen B, Hoffer K, Struve N, Myllynen L, Braig F, Binder M, Rieckmann T, Grénman R, Petersen C, Dikomey E, Rothkamm K. Radiosensitization of HNSCC cells by EGFR inhibition depends on the induction of cell cycle arrests. Oncotarget, 2016, 7(29):45122-45133.
- Möckelmann N, Rieckmann T, Busch CJ, Becker B, Gleissner L, Hoffer K, Omniczynski M, Steinmeister L, Laban S, Grenman R, Petersen C, Rothkamm K, Dikomey E, Knecht R, Kriegs M. Effect of sorafenib on cisplatin-based chemoradiation in head and neck cancer cells. Oncotarget, 2016, ;7(17):23542-51.
- Braig F, Voigtlaender M, Schieferdecker A, Busch C-J, Laban S, Grob T, Kriegs M, Knecht R, Bokemeyer C, Binder M. Liquid biopsy monitoring uncovers acquired RAS-mediated resistance to cetuximab in a substantial proportion of patients with head and neck squamous cell carcinoma. Oncotarget, 2016, doi: 10.18632/oncotarget.8943.
- Busch C-J, Becker B, Kriegs M, Gatzemeier F, Krüger K, Möckelmann N, Fritz G, Petersen C, Knecht R, Rothkamm K. Similar cisplatin sensitivity of HPV-positive and -negative HNSCC cell lines. Oncotarget, 2016, 7(24):35832-35842.
- Myllynen L*, Kwiatkowski M*, Gleißner L*, Riepen B, Hoffer K, Wurlitzer M, Petersen C, Dikomey E, Rothkamm K, Schlüter H**, Kriegs M**. *shared-first authorship; **shared-last authorship. Quantitative proteomics unveiled: Regulation of DNA double strand break repair by EGFR involves PARP1. Radiother Oncol, 2015,116(3):423-30.
- Güster JD*, Weissleder SV*, Busch CJ, Kriegs M, Petersen C, Knecht R, Dikomey E, Rieckmann T, *shared-first authorship. The inhibition of PARP but not EGFR results in the radiosensitization of HPV/p16-positive HNSCC cell lines. Radiother Oncol, 2014, 113(3):345-51.
- Laban S*, Steinmeister L*, Grob TJ, Grénman R, Petersen C, Gal A, Knecht R, Dikomey E, Kriegs M, *shared-first authorship. Sorafenib sensitizes head and neck squamous cell carcinoma cells to ionizing radiation. Radiother Oncol, 2013, 109(2):286-92.
- Saker J, Kriegs M, Zenker M, Heldt JM, Eke I, Pietzsch HJ, Grénman R, Cordes N, Petersen C, Baumann M, Steinbach J, Dikomey E, Kasten-Pisula U. Inactivation of HNSCC cells by 90Y-labeled Cetuximab strictly depends on the number of induced DNA double strand breaks. J Nucl Med, 2013, 54(3):416-23.
- Busch CJ, Kriegs M, Laban S, Tribius S, Knecht R, Petersen C, Dikomey E, Rieckmann T. HPV-positive HNSCC cell lines but not primary human fibroblasts are radiosensitized by the inhibition of Chk1. Radiother Oncol; 2013. Sep;108(3):495-9.
- Kasten-Pisula U, Saker J, Eicheler W, Krause M, Yaromina A, Meyer-Staeckling S, Scherkl B, Kriegs M, Brandt B, Grénman R, Petersen C, Baumann M, Dikomey E. Cellular and tumor radiosensitivity is correlated to epidermal growth factor receptor protein expression level in tumors without EGFR amplification. Int J Radiat Oncol Biol Phys, 2011, 15;80(4):1181-8.
Project 2 (PI: Struve):
Epidermal growth factor variant III (EGFRvII) in Glioblastoma (GBM)
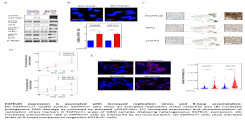
This project focuses on Glioblastoma Multiforme (GBM), which is an aggressive primary brain tumor, characterized by rapid progression and oncogene activation. One common and frequently expressed oncogene in GBM is the constitutively activated epidermal growth factor receptor deletion mutant EGFRvIII. EGFRvIII represents a typical oncoprotein that activates different signaling pathways altering thereby multiple cellular processes. Therefore, analyzing how EGFRvIII impacts therapy relevant processes like DNA repair, replication, transcription and cellular signaling are the main research interests. By understanding these complex processes, we hope to identify specific and new targeting approaches to individualize GBM therapy and to finally form the molecular rationale to improve overall survival.
EGFRvIII expression is associated with increased replication stress and R-loop accumulation: (A) EGFRvIII-/+ model system: EGFRvIII+ cells show an activated replication stress response and (B) increased endogenous DNA damage as indicated by elevated γH2AX-foci. (C) Increased expression and phosphorylation of replication stress markers in EGFRvIII+ areas of GBM samples displaying heterogeneous EGFRvIII expression. (D) Increased transcription rate in EGFRvIII+ cells as measured by EU-incorporation. (E) EGFRvIII+ cells show elevated levels of R-loops compared to isogenetic EGFRvIII- cells
Contact: [email protected]
Collaboration: Prof. Dr. Ulrich Schüller, Kinderkrebs-Zentrum Hamburg & Institut für Neuropathologie, UKE; Prof. Dr. Susan Short, Leeds University, UK; Prof. Dr. Hartmut Schlüter, Mass Spectrometric Proteomics, Institut für Klinische Chemie, UKE; Prof. Dr. Michael Hausmann, Kirchhoff-Institut für Physik, Universität Heidelberg; PD Dr. Kristian Unger, Helmholzt-Zentrum München.
Grants: Wilhelm-Sander Stiftung; Brigitte & Dr. Konstanze Wegener-Stiftung; Forschungs- und Wissenschaftsstiftung Hamburg (Landesexzellenzinitiative); Forschungsförderungsfonds des Fachbereichs Medizin
Publications:
- Struve N, Brend T, Stead LF, Binder ZA, Bagley SJ, Faulkner C, Ott L, Müller-Goebel J, Hoffer K, Morrissette JDJ, Schüller U, Petersen C, Rothkamm K, Kurian KM, O´ Rourke DM, Short SC, Kriegs M. EGFRvIII improves temozolomide response in MGMT promoter methylated glioblastoma via upregulation of DNA mismatch repair. Oncogene. 2020 Apr;39(15):3041-3055.
- Luger AL, Lorenz NI, Urban H, Divé I, Engel AL, Strassheimer F, Dettmer K, Zeiner PS, Shaid S, Struve N, Kriegs M, Hofmann U , Oefner PJ, Harter PN, Steinbach JP, Ronellenfitsch MW. Activation of Epidermal Growth Factor Receptor Sensitizes Glioblastoma Cells to Hypoxia-Induced Cell Death. Cancers. 2020, 12, 2144.
- Ketchen S, Rohwedder A, Knipp S, Esteves F, Struve N, Peckham M, Ladbury JE, Curd A, Short SC, Brüning-Richardson A. A novel workflow for three-dimensional analysis of tumour cell migration. Interface Focus. 2020 Apr 6;10(2):20190070.
- Harmer J, Struve N, Brüning-Richardson A. Characterization of the Effects of Migrastatic Inhibitors on 3D Tumor Spheroid Invasion by High-resolution Confocal Microscopy. J Vis Exp. 2019 Sep 16;(151).
- Boyd PS*, Struve N*, Bach M, Eberle JP, Gote M, Schock F, Cremer C, Kriegs M**, Hausmann M**. *shared-first authorship; **shared-last authorship. Clustered localization of EGFRvIII in glioblastoma cells as detected by high precision localization microscopy. Nanoscale, 2016, 28;8(48):20037-20047.
- Riedel M*, Struve N*, Müller-Göbel J, Köcher, S Petersen C, Dikomey E, Rothkamm K, Kriegs M. Sorafenib inhibits cell growth, but fails to enhance radio- and chemosensitivity of glioblastoma cell lines. *shared-first authorship. Oncotarget, 2016, 7(38):61988-61995.
- Struve N, Riedel M, Schulte A, Rieckmann T, Grob TJ, Gal A, Rothkamm K, Lamszus K, Petersen C, Dikomey E, Kriegs M. EGFRvIII does not affect radiosensitivity with or without gefitinib treatment in glioblastoma cells. Oncotarget, 2015, 20;6(32):33867-77.
Projekt 3 (PI: Kriegs, Struve):
Epigenetic and kinome-spezific patterns for predicting the response of glioblastomas to X-rays and temozolomide
This project is part of the graduate school "Innovative Technologies in Cancer Diagnostics and Therapy" which starts in October 2020.
Glioblastoma (GBM) is one of the most common and aggressive brain tumours. GBM is treated by means of resection, radiotherapy and temozolomide (TMZ). However, there are great differences in the response to the various treatment modalities, although the mechanisms are largely unknown, especially with regard to radiation sensitivity. The primary aim is to identify patterns in the methylom and kinome of GBM cells associated with radiation and TMZ sensitivity. Both basal and radiation- or TMZ-induced patterns will be analysed. Furthermore, the patterns of resistance and sensitivity will be tested on patient material and compared with the response to therapy.
Contact: [email protected]
Collaborations: Prof. Dr. Ulrich Schüller, Institut für Neuropathologie & Kinderkrebs-Zentrum Hamburg
Grants: Landesforschungsförderung der Hansestadt Hamburg
Project 4 (PI: Bußmann):
Predictive marker for immune checkpoint inhibition in HNSCC
This project started in August 2020. The aim of this project is to identify responder and non-responder to immune checkpoint inhibition (ICI) to guide further therapy of head and neck squamous cell carcinoma (HNSCC) patients.
Immune checkpoint inhibitors have already been approved as 1st and 2nd line therapy of relapsed or distant metastatic head and neck squamous cell carcinoma (R/M-HNSCC). However, only 13-18% respond to therapy with no definitive predictive markers available resulting in a great need to establish predictive tests identifying responding and non-responding patients.
For ICI therapy of advanced malignant melanoma and non small cell lung carcinoma it has been demonstrated, that specific kinase activity clusters of peripheral blood mononuclear cells (PBMC) can be predictive. These clusters result from functional kinome profiling and might be promising in R/M-HNSCC. To test this, liquid biopsies will be collected from HNSCC patients before and under ICI treatment. PBMC will be isolated and functional kinome profiling will be performed. After cluster analysis the kinase activity clusters of the PBMC will be compared with the survival data of the patients.
This method may also allow to monitor the patients` individual response during ICI treatment enabling the early detection of a progresses. Such a predictive test would support to identify responding versus non-responding patients and to guide non-responders to a more appropriate therapy. This would also enable a more efficient economical use of the expensive therapeutics.
In the future, such predictive test may also be transferred to additional entities.
Contact: [email protected]
Collaborations: Department of Otorhinolaryngology; UCCH-Biobank; Marienkrankenhaus Hamburg: Department of Oncology (Prof. Dr. med. U. Vanhoefer/Dr. med. G. Hapke), Department of Otorhinolaryngologie (PD Dr. A. Münscher); PamGene International BV (The Netherlands)
Grants: Mildred Scheel Cancer Career Center HaTriCS4, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; Brigitte und Dr. Konstanze Wegener-Stiftung
UCCH Kinomics Core Facility (PI: Kriegs, Bußmann)
The UCCH Kinomics Core Facilitiy offers functional kinome profiling using a PamStation®12 from PamGene Int. (The Netherlands). The PamTechnology is a microarray-based system which consists PamChips® holding peptides specific for either serine/threonine (STK Chips) or tyrosine kinases (PTK Chips). Sequence specific phosphorylation of the peptides by the kinases within a lysate firstly provides information on the overall kinase activity (A). Subsequent bioinformatic prediction methods (so-called upstream kinase analyses, Bionavigator software) identify the most prominent kinases (B). The technique deduces the activity of the majority of the approx. 500 kinases within the human kinome.
Contact: [email protected]
See also: www.uke.de/english/departments-institutes/centers/university-cancer-center-hamburg-(ucch)%E2%80%94hubertus-wald-tumor-center/research/core-facilities/ucch-research-core-facilities-details.html
Kinome profiling using the PamGene's microarray assay. (A) The PamGene's microarray assay for kinase activity profiling is based on measuring peptide phosphorylation by protein kinases. The PamChip® disposable consists of 4 identical arrays, each array containing up to 144 (STK) or 196 (PTK) peptides immobilized on a porous ceramic membrane. The peptide sequences (13 amino acids long) harbor phosphorylation sites derived from literature or computational predictions and are correlated with one or multiple upstream kinases. Fluorescently labelled anti-phospho antibodies are used to detect phosphorylation activity of kinases present in the sample. (C) During the assay, the sample solution is pumped through the porous membrane, allowing for faster kinetics and real-time measurements. Images are taken for each array at several exposure times by the CCD camera in the workstation. (BD) Images can later used by the BioNavigator® software to generate heat maps and upstream kinase prediction. The data workflow consistis of image quantification, quality control, statistical analysis, visualization and interpretation.
Collaborations:
PamGene International BV (The Netherlands)
Publications:
- Dyshlovoy SA, Kaune M, Kriegs M, Hauschild J, Busenbender T, Shubina LK, Makarieva TN, Hoffer K, Bokemeyer C, Graefen M, Stonik VA,von Amsberg G. Marine alkaloid monanchoxymycalin C: a new specific activator of JNK1/2 kinase with anticancer properties. Sci Rep. 2020 Aug 6;10(1):13178.
- Tintelnot T, Metz S, Trentmann M, Oberle A, Braig F, Kriegs M, Fehse B, Riecken K, Bokemeyer C, Stein A, Binder M. Cancer cells expressing oncogenic Rat sarcoma show drug-addiction towards epidermal growth factor receptor antibodies mediated by sustained mitogen-activated protein kinase signaling. Front Oncol. 2020 Jan 21;9:1559.
- Struve N, Binder ZA, Stead LF, Brend T, Bagley SJ, Faulkner C, Ott L, Müller-Goebel J, Weik AS, Hoffer K, Krug L, Rieckmann T, Bußmann L, Henze M, Morrissette JJD, Kurian KM, Schüller U, Petersen C, Rothkamm K, O Rourke DM, Short SC, Kriegs M. EGFRvIII improves temozolomide response in MGMT promoter methylated glioblastoma via upregulation of DNA mismatch repair. Oncogene. 2020 Apr;39(15):3041-3055..
- Hoorens M, Ourailidou ME, Rodat T, Wouden Pvd, Kobauri P, Kriegs M, Peifer C, Feringa BL, Dekker FD, Szymanski W. Light-controlled Inhibition of BRAFV600E Kinase. Eur J Med Chem. 2019 Oct 1;179:133-146.
- Peifer C, Schmidt D, Rodat T, Heintze L, Weber J, Horbert R, Girreser U, Raeker T, Bußmann L, Kriegs M, Hartke B. Axitinib - A Photoswitchable Approved Tyrosine Kinase Inhibitor. ChemMedChem. 2018 Nov 20;13(22):2415-2426.
5. Fellowships & Awards:
2020 Research award of the Hamburger Cancer Society (Struve/Kriegs)
2020 Hubertus Wald Junior Investigator Award 2020 (Struve)
2020 MSNZ Clinician Scientist (Bußmann)
2019 MD stipend E.W. Kuhlmann-Stiftung (Cetin)
2018 UCCH Clinician Scientist (Berger, alumna)
2017 UCCH Clinician Scientist (Bußmann)
2016 MD stipend Werner Otto-Stiftung (Struve/Müller-Goebel, alumnus)
2016 Friedrich Zywietz-Promotionspreis (Struve)
2016 UCCH Clinician Scientist (Bußmann)
2015 UKE International Travel Scholarships (Kriegs/Struve)
2015 GlaxoSmithKline Stiftung Reisestipendium (Struve)
2015 Oncoray Poster Prize (Gleißner, alumna)
2014 Friedrich Zywitz-Promotionspreis (Myllynen, alumna)
2013 UCCH Clinician Scientist (Möckelmann, alumnus)
2012 UCCH Clinician Scientist (Busch, alumna)
2011 UCCH Clinician Scientist (Laban, alumnus)